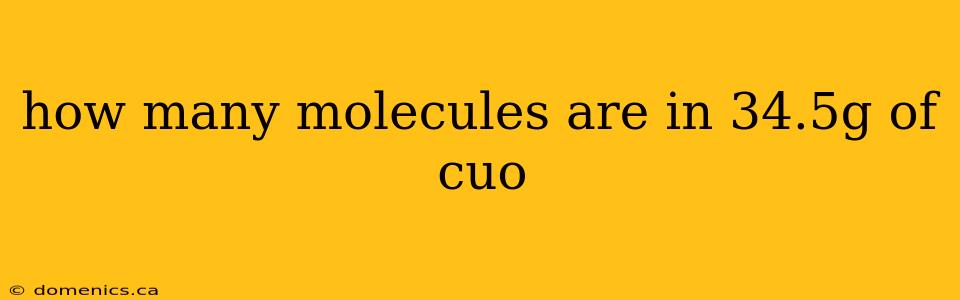
How Many Molecules Are in 34.5g of CuO? A Step-by-Step Guide
Determining the number of molecules in a given mass of a substance involves a few key steps. Let's break down how to calculate the number of molecules in 34.5g of copper(II) oxide (CuO).
Understanding the Fundamentals
Before diving into the calculation, we need to understand some fundamental concepts:
-
Molar Mass: The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's essentially the sum of the atomic masses of all the atoms in a molecule. We'll need the periodic table to find the atomic masses of copper (Cu) and oxygen (O).
-
Avogadro's Number: This is a fundamental constant in chemistry, representing the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. Avogadro's number is approximately 6.022 x 10²³.
-
Mole Concept: A mole is a unit of measurement that represents a specific number of particles (Avogadro's number). It's the bridge between the macroscopic world (grams) and the microscopic world (number of molecules).
1. Calculate the Molar Mass of CuO
First, we need to determine the molar mass of CuO. Using a periodic table:
- The atomic mass of copper (Cu) is approximately 63.55 g/mol.
- The atomic mass of oxygen (O) is approximately 16.00 g/mol.
Therefore, the molar mass of CuO is: 63.55 g/mol + 16.00 g/mol = 79.55 g/mol
2. Convert Grams to Moles
Next, we convert the given mass of CuO (34.5g) into moles using the molar mass:
Moles of CuO = (Mass of CuO) / (Molar mass of CuO) Moles of CuO = 34.5 g / 79.55 g/mol ≈ 0.433 moles
3. Calculate the Number of Molecules
Finally, we use Avogadro's number to determine the number of molecules:
Number of molecules = (Moles of CuO) x (Avogadro's number) Number of molecules = 0.433 moles x 6.022 x 10²³ molecules/mol ≈ 2.61 x 10²³ molecules
Therefore, there are approximately 2.61 x 10²³ molecules in 34.5g of CuO.
Important Considerations:
-
Significant Figures: The final answer should reflect the significant figures in the given data. In this case, 34.5g has three significant figures, so our answer is rounded to three significant figures as well.
-
Accuracy: The atomic masses used from the periodic table are approximations. Slight variations in the values may lead to minor differences in the final result.
This step-by-step process demonstrates how to calculate the number of molecules in a given mass of any compound. Remember to always use the correct molar mass and Avogadro's number for accurate results. This method is crucial in various chemical calculations and provides a fundamental understanding of the relationship between mass, moles, and the number of molecules.